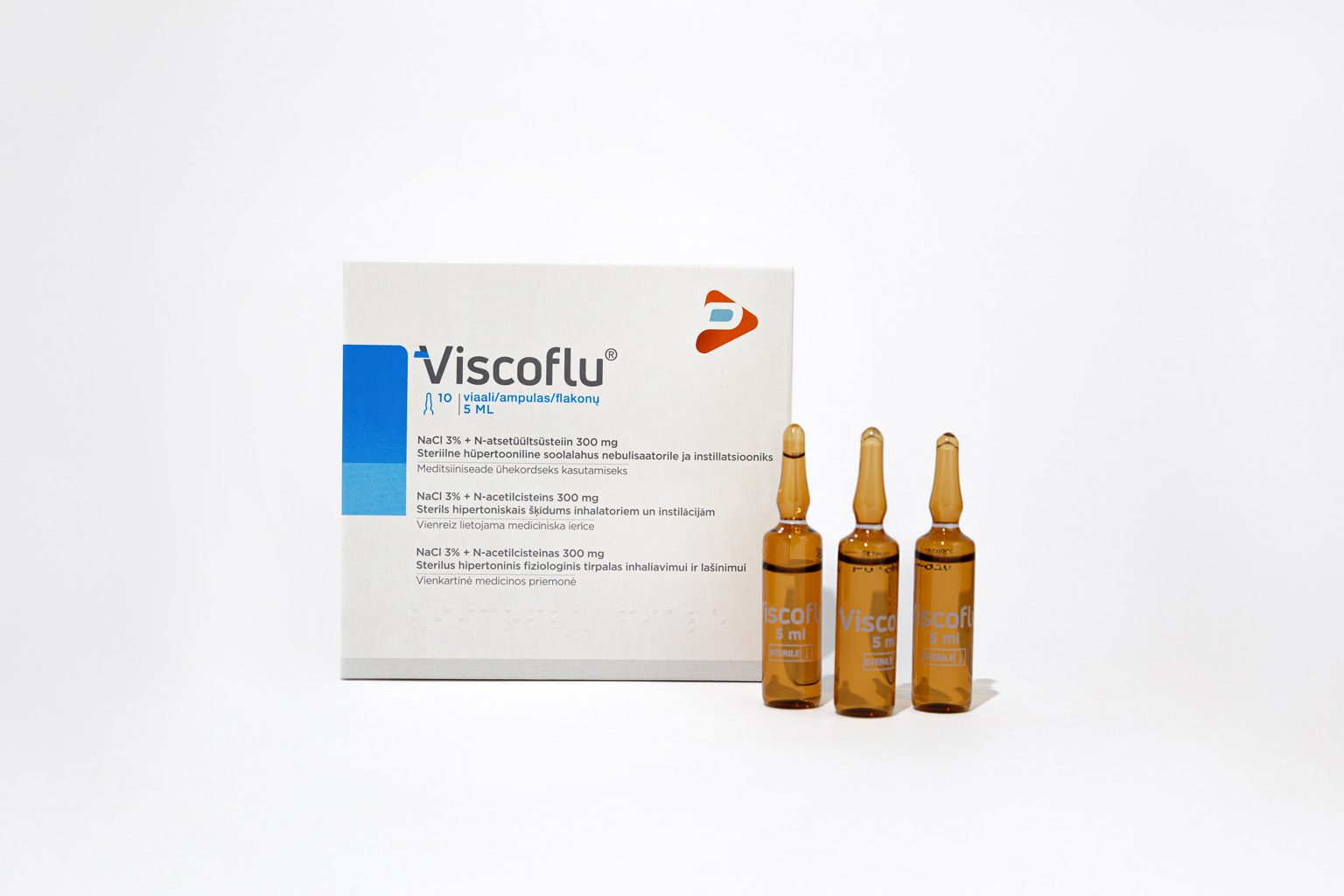
Viscoflu® is a single-use medical device indicated during treatment of respiratory diseases featuring dense and viscous hypersecretion: acute bronchitis, chronic bronchitis and its complications, pulmonary emphysema, cystic fibrosis, bronchiectasis, sinusitis, secretory otitis.
Contraindications and limitations for use
Hypersensitivity to the active ingredients or to any of the excipients.
Viscoflu® is contraindicated in children under 2 years of age.
Viscoflu® should only be administered during pregnancy and breastfeeding if it is absolutely necessary and under the direct super¬vision of a doctor.
Mechanism of action
Viscoflu® thins dense and viscous mucus through the physical/ mechanical action of its ingredients:
• sterile buffered hypertonic solution of sodium chloride: hyper¬tonic saline solutions applied directly to a mucus membrane by osmosis draw water from the intracellular to the extracellular compartment. This increases the percentage of water in the mucus lining the epithelium, thinning it and facilitating its removal;
• N-acetylcysteine (NAC): acetylated amino acid cysteine variant, exerts a mucolytic activity by direct contact with the mucus. This activity depends on the presence in the molecule of sulphydryl radicals capable of breaking the disulfide bonds of the mucoproteins that give mucus viscosity, depolymerising it. NAC also has an antioxidant action that helps preserve the tropism of the respiratory epithelia.
Packaging format
• wallet containing 5 ampoules of 5 ml each
• wallet containing 10 ampoules of 5 ml each
Method of use
Viscoflu® is a sterile solution that can be:
• nebulized by aerosol dispensers to be inhaled in order to reach the bronchial region;
• directly instilled by ear and nose wash;
• instilled at the endobronchial level through permanent catheters, bronchoscopy, etc.;
Overdose
Excessive doses of Viscoflu® by inhalation and endotrache¬al-bronchial instillation could lead to massive and excessive fluid secretions that, in patients with depression of the cough reflex and expectoration in particular, may make bronchoalveolar lavage ne¬cessary.
Known side effects
Due to the high salt concentration of the solution, the use of Vi¬scoflu® can induce occasional localised irritation phenomena that can cause bronchospasm, burning sensation in the nasal mucosa, rhinorrhea, nausea, vomiting. Among these effects, the induction of the cough reflex within tolerable limits is to be welcomed as it promotes expectoration and unblocking of airways.The side effects of Viscoflu® usually disappear spontaneously by discontinuing tre¬atment
If they do not disappear, consult your doctor. Following the instructions in the leaflet reduces the risk of side effects.
Known interactions with other medical devices or drugs
In patients treated with nitroglycerin-based drugs it is appropriate to consult a doctor before using Viscoflu® because the presence of N-acetylcysteine may cause hypotension with dilation of the tempo¬ral artery and the possible occurrence of headache: pressure should be monitored in this case.
Viscoflu® should not be administered with anti-cough drugs becau¬se the reduction of the cough reflex may lead to the accumulation of bronchial secretions.
Viscoflu® can be administered together with bronchodilators, va¬soconstrictors, etc., but should in this case be used for the shortest time possible.
It is not recommended to mix antibiotics with Viscoflu® solution.The N-acetylcysteine contained in Viscoflu® can interfere with the test for measuring salicylates and ketones in the urine.
You are recommended to inform your doctor or pharmacist before using Viscoflu® if you have recently taken any other drugs, including drugs obtained without prescription.
Storage conditions
It is recommended to open the Viscoflu® ampoule just before use: open ampoules can be used only when stored in a refrigerator for a maximum of 24 hours. If the solution has been mixed with other drugs, it should be used in the shortest possible time and cannot be stored. The expiry date printed on the packaging refers to the uno¬pened pack, correctly stored. Do not use Viscoflu® after the expiry date printed on the packaging. Keep it out of the reach and sight of children.
Warning
When opening the ampoule Viscoflu® emits a sulphurous smell which does not affect the administration of the preparation in any way. The solution can sometimes turn a pink colour in the open ampoule or when drawn into the aerosol device, without compro¬mising the activity and tolerability of the preparation. If you have any further questions on the use of Viscoflu® please contact your doctor or pharmacist. Patients with bronchial asthma must be clo¬sely monitored during therapy. If bronchospasm arises, the admini-stration of N-acetylcysteine should be immediately suspended, and an appropriate treatment must be carried out.
Manufacturer: Pharma Line S.r.l., Italy